How MEDDEV 2.7.1 Rev 4 Affects Medical Device Manufacturers

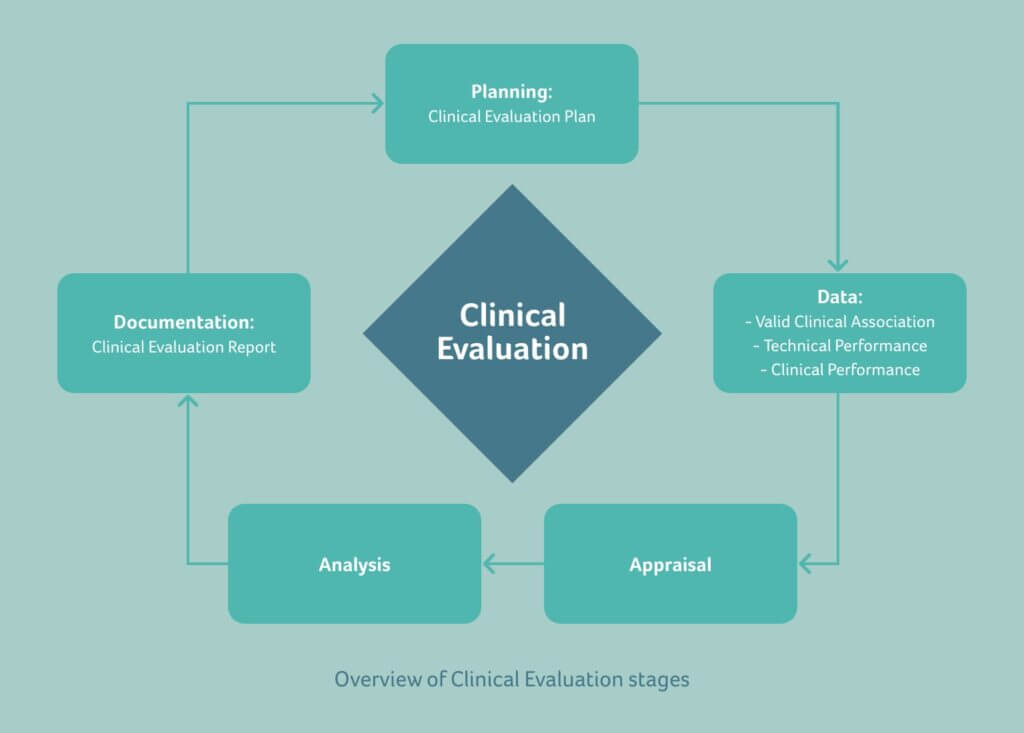
Clinical Evaluation - Compliance to MEDDEV 2.7/1 Rev 4 and MDR 2017/745. Clinical Evaluation requirements have increased dramatically since the release of MEDDEV 2.7.1 Rev 4 in 2016 and the MDR 2017/745 in May of 2017. The process now involves two documents; the Clinical Evaluation Plan (CEP) and Clinical Evaluation Report (CER).
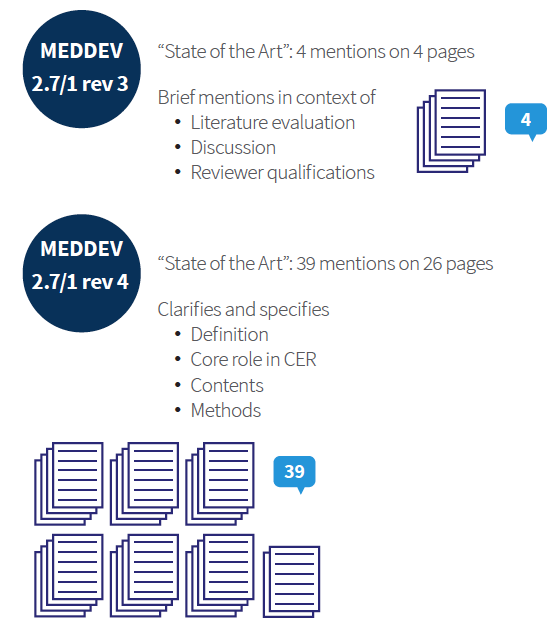
Has MEDDEV 2.7/1 rev 4 changed the state of the art requirements?
MEDDEV 2.7 1 Rev 4: Conducting a Clinical Evaluation General principles of clinical evaluation The objective of the clinical evaluation is to prove that the product is safe and effective based on a set of clinical data, i.e. "the safety and/or performance information that is generated from the clinical use of a device" (MDD 93/42 EWG.
Checkliste So gelingt die Literaturrecherche nach MEDDEV 2.7.1 rev. 4 und MDR

MEDDEV 2.5/7 rev.1 (92 kB) Conformity assessment of breast implants . July 1998. MEDDEV 2.5/9 rev.1 (96 kB) Evaluation of medical devices. The new SAE reporting form was taken in use by 1 September 2016. MEDDEV 2.7/4 (183 kB) Guidelines on clinical investigations: a guide for manufacturers and notified bodies . December 2010 2.10 Notified
MDD指南,MEDDEV 2.121 rev.82013.01, 医疗器械警戒系统指南_文档之家
The European Commission has published a guide on clinical evaluation under the directives 93/42/EEC and 90/385/EEC; MEDDEV 2.7/1 rev. 45. This MEDDEV guide should be used also during the process. MDCG 2020-5 Clinical Evaluation - Equivalence. A guide for manufacturers and notified bodies.
Die MEDDEV 2.7/1 Revision 4 Leitfaden für klinische Bewertungen
Telephone: (32-2)299.11.11. Fax: (32-2) 296 70 13. E-mail: entr-medical-devices@cec.eu.int EUROPEAN COMMISSION ENTERPRISE DIRECTORATE-GENERAL Single Market : regulatory environment, standardisation and New Approach Pressure equipment, medical devices, metrology MEDDEV. 2.7.1 April 2003 GUIDELINES ON MEDICAL DEVICES EVALUATION OF CLINICAL DATA :
Medical Device Equivalence How Close Does It Need to Be Under the EU MDR and MEDDEV 2.7/1 rev 4

How does the European medical devices regulation (MDR 2017/745) impact CER requirements? MDR 2017/745 and a revised CER guidance (MEDDEV 2.7/1 rev 4) both reflect more stringent requirements for clinical data. The MDR became fully applicable in Europe in May 2021.
(MEDDEV) 2.7.1 rev.4 CLINICAL EVALUATION

2014 NBOG BPG 2014-2 2016 MedDev 2.7/1 rev 4 Further expansion of the guidance, to reinforce concepts around quality and completeness of clinical data and scientific validity of conclusions (increase from 46 to 65 pages) 2017 Regulation (EU) 2017/745 Clinical evaluation requirements largely aligned with MedDev 2.7/1 rev 4 become enshrined in EU law
医疗器械警戒系统指南MEDDEV 2.121 REV.8 January 2013_文档之家
In June 2016, the MEDDEV 2.7.1 rev 4, the European guidance on the clinical evaluation of medical devices (MD) was published. The issuance of this guidance happened shortly after the publication of the text of the future Regulation on the Medical Device (MDR) of 15 June 2016, which anticipates many new requirements for clinical evaluation..
Comparison of key definitions in MEDDEV 2.7/1 Rev 4 and the MDR Download Scientific Diagram

The most important aspects to remember when documenting the literature search are documented in Annex 5 of 2.7 1 rev 4. The MEDDEV 2.7 1 rev 4 focusses on the following sections of Literature search and review: Search categories (e.g. medical device search or state of the art including clinical condition), Scope of the search strategy
MEDDEV 2.7.1 Rev 4 New Requirements and Changes for Clinical Evaluation Reports (CER) YouTube

- MEDDEV 2.12/1 Guidelines on a medical devices vigilance system - MEDDEV 2.12/2 Guidelines on post market clinical follow-up studies: a guide for manufacturer and notified body - MEDDEV 2.4/1 Classification of medical devices - MEDDEV 2.7/2 Guidelines for competent authorities for making a validation/assessment of a
Navigating Clinical Evaluation for SaMD Congenius
If you are interested in learning more about creating a quality plan for compliance with MEDDEV 2.7/1 Rev 4, or need help in updating your clinical evaluations, please contact our team today. contact@pearlpathways.com. 317.602.5479 www.pearlpathways.com @PearlPathways.
医疗器械警戒系统指南MEDDEV 2.121 REV.8 January 2013_文档之家
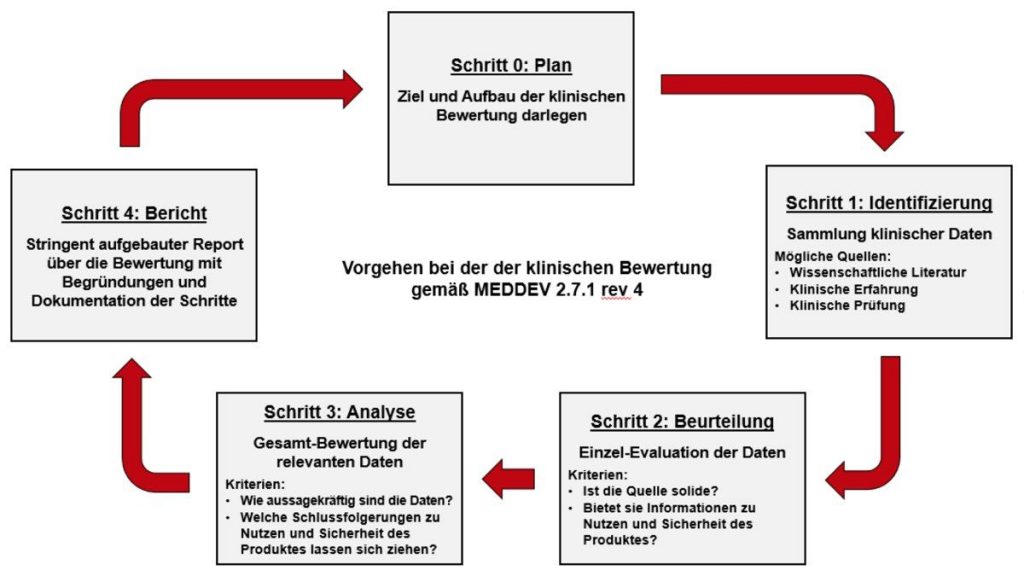
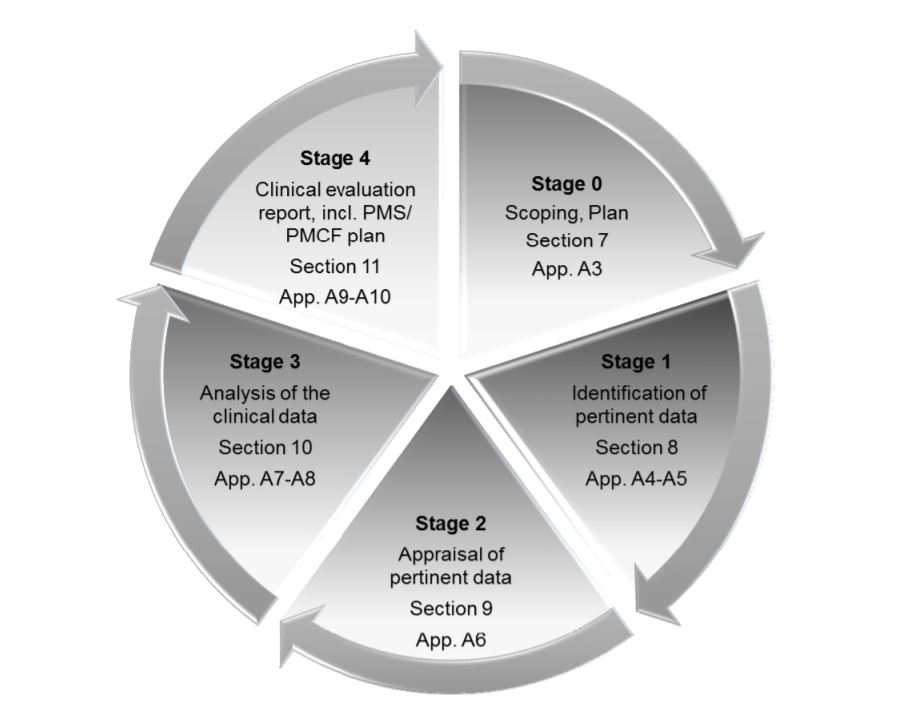
MEDDEV 2.7.1 Rev. 4 is the guideline for performing the clinical evaluation of a medical device before CE marking and after the CE marked stage. It has a stage-wise explanation for the clinical assessment. Stage 0 contains the Scoping and the planning of the Clinical evaluation, which specifies the contents as below: Device description.
The top ten changes in MEDDEV 2.7.1 Rev 4
The full title of MEDDEV 2.7/1 Rev. 4 is "Clinical Evaluation: A Guide for Manufacturers and Notified Bodies under Directives 93/42 EEC and 90/385/EEC.". The reference to the MDD and AIMDD should be a big clue that this document is not aligned with EU MDR. However, while the regulations governing clinical evaluation have changed, the.
EU MDR Vigilance Reporting Requirements and MEDDEV 2.121 Rev 8 Oriel STAT A MATRIX Blog

Background note on the relationship between MDCG 2020-6 and MEDDEV 2.7/1 rev.4 on clinical evaluation. April 2020: MDCG 2020-5: Guidance on clinical evaluation - Equivalence: April 2020: MDCG 2019-9 - Rev.1: Summary of safety and clinical performance: March 2022
如何编写或者更新CE第四版临床评价报告MEDDEV 2.7/1 Rev. 4 知乎

MEDDEV 2.7.1 Rev 4: Key changes and clarifications BSI MEDDEV 2.7.1 Rev 4 top 10 changes Call us now on 1 800 862 4977 Clarification: Frequency of updates to the Clinical Evaluation Report (CER). Clause 6.2.3 requires the CER to be updated at least annually for high risk or new devices, and every 2 to 5 years
What is a Clinical Evaluation Report? The Kolabtree Blog
MEDDEV 2.7/1.rev. 4 (COM 2016) specifies requirements for the professional qualifications, knowledge, and declarations of interest of clinical evaluators (individuals or teams) to ensure an independent and competent clinical evaluation. The manufacturer must formulate requirements for clinical evaluators based on the nature, intended use.